
New Drug Designations - November 2024
Shots:
- PharmaShots' designation report provides a concise overview of the latest drug designations by major regulatory authorities, including the FDA, EMA, and NMPA
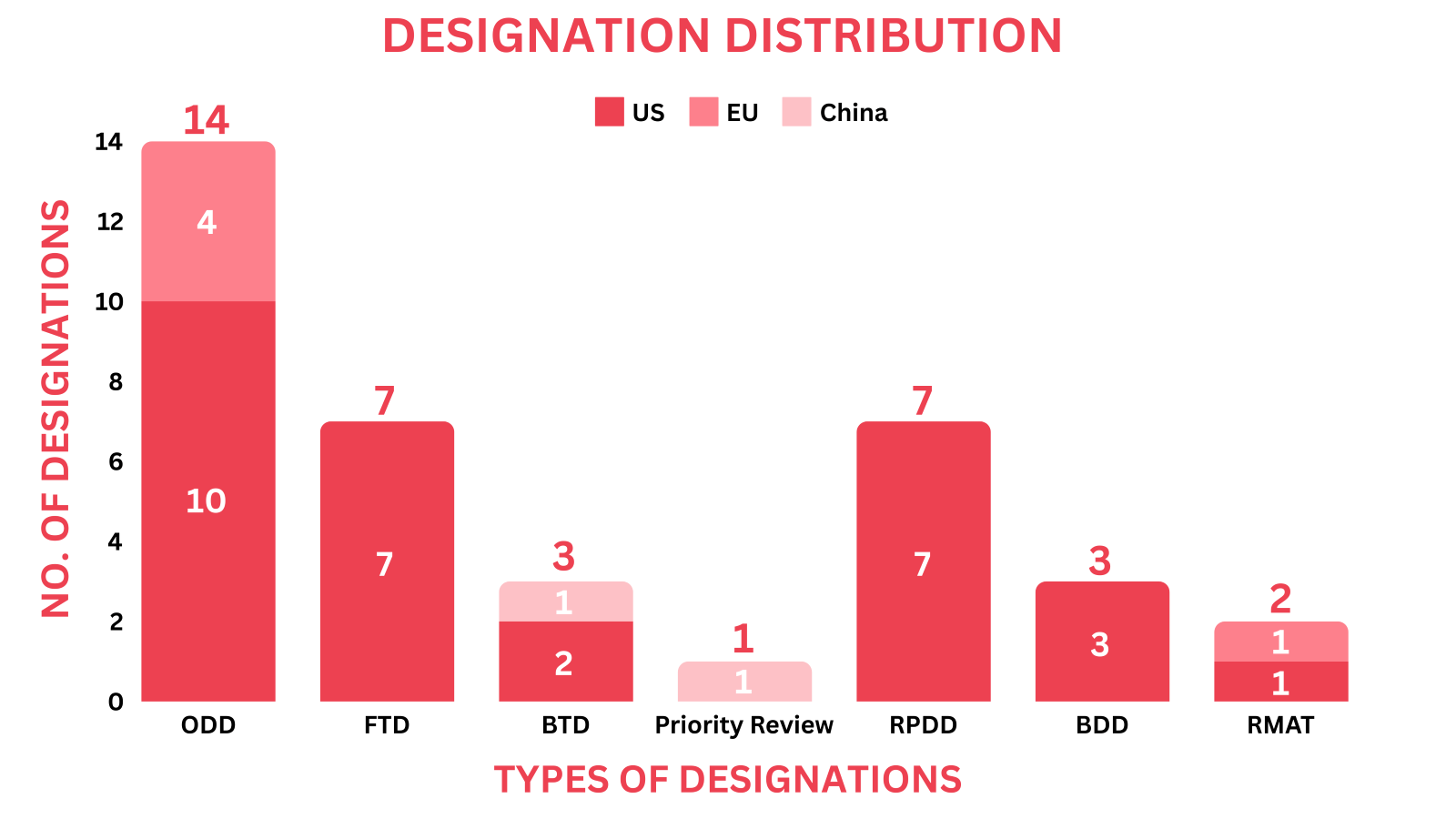
- The November 2024 report covers designations granted to 28 drugs and 3 devices, encompassing 9 small molecules, 6 biologics, 7 cell and gene therapies as well as 3 medical devices
- Significant trends this month show, Cumberland Pharmaceuticals’ Ifetroban secured the FDA's ODD and RPDD for the treatment of cardiomyopathy related to Duchenne muscular dystrophy (DMD)

LBL-024
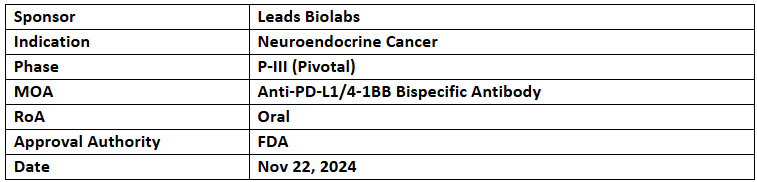
- The US FDA granted Orphan Drug Designation (ODD) to LBL-024 for the treatment of neuroendocrine cancer. The drug also received Breakthrough Therapy Designation (BTD) from the NMPA for the same indication
- LBL-024 secured Investigational New Drug (IND) approvals from the US FDA and NMPA in 2021 for P-I/II trials. The drug demonstrated more than double the Objective Response Rate (ORR) and Overall Survival (OS) compared to existing treatments. It is currently being studied in a single-arm pivotal trial for extrapulmonary neuroendocrine carcinomas
- LBL-024 is a tetravalent bispecific antibody targeting PD-L1 and 4-1BB to inhibit immune suppression and enhance T-cell activation. This mechanism synergistically boosts anti-tumor activity.
Ifetroban

- The US FDA has granted ODD and Rare Pediatric Disease Designation (RPDD) to Ifetroban for the treatment of cardiomyopathy associated with Duchenne muscular dystrophy (DMD)
- The company is conducting an additional P-II study (FIGHTING FIBROSIS) to assess the safety and efficacy of Ifetroban compared to placebo (PBO) in patients with idiopathic pulmonary fibrosis (IPF) over a 52-week treatment period
- Ifetroban (QD, oral) is a selective thromboxane-prostanoid receptor (TPr) antagonist currently being evaluated in a P-II study (FIGHT DMD) for its safety, efficacy, and pharmacokinetics (PK) in patients with DMD, with results expected in Q4 2024.
AT-02
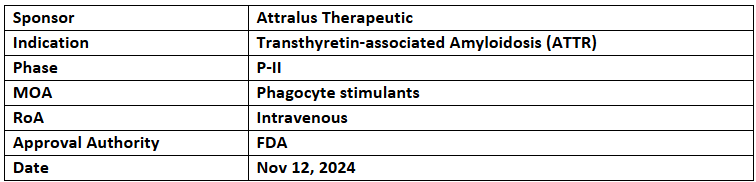
- The US FDA has granted ODD to AT-02 (IgG1) for the treatment of transthyretin-associated amyloidosis (ATTR)
- AT-02, the company’s lead pan-amyloid removal candidate (PAR), is being evaluated in a three-part P-I & P-II open-label extension trial for systemic amyloidosis
- Earlier this year, the EMA's COMP recommended AT-02 for the treatment of ATTR and AL amyloidosis, with the EC adopting these recommendations on Aug 21, 2024.
Neflamapimod
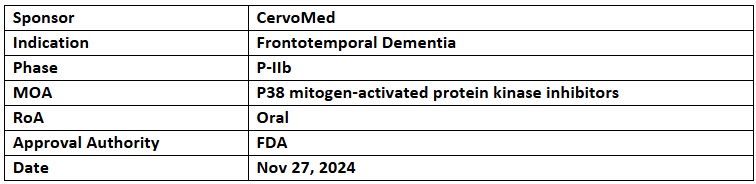
- The US FDA has granted ODD to Neflamapimod for the treatment of frontotemporal dementia (FTD)
- Neflamapimod (40 mg, oral, TID) is under investigation in a P-IIb (RewinD-LB) trial for early-stage Dementia with Lewy bodies (DLB) patients (n=159), with topline data expected in Dec 2024. Discussions are ongoing for a proof-of-principle study in FTD, and preparations for a P-III trial in DLB are planned for mid-2025
- Neflamapimod reversed synaptic dysfunction in the basal forebrain in preclinical studies. It demonstrated good tolerability in P-I & P-II trials involving over 300 subjects. The P-IIa (AscenD-LB) study reported improvements in dementia severity, functional mobility, and cognitive function.
LBL-034
- The US FDA has granted ODD to LBL-034 for the treatment of multiple myeloma (MM). It is developed by Leads Biolabs using its proprietary T-cell engager antibody technology platform, LeadsBody
- Leads Biolabs received IND approvals from the NMPA and FDA in July 2023 and initiated a P-I/II dose-escalation/expansion trial of LBL-034 for relapsed/refractory (r/r) MM in Nov 2023 across China. Preliminary data showed favorable safety and strong efficacy. These findings will be presented at ASH 2024
- LBL-034 is a novel bispecific antibody targeting CD3 on T cells and GPRC5D on cancer cells to induce cancer cell destruction. It demonstrated high binding affinity, strong potency, and a reduced risk of T-cell exhaustion, indicating best-in-class potential.
AB-1003
- The US FDA has granted ODD and RPDD to AB-1003 (LION-101) for the treatment of Limb-Girdle Muscular Dystrophy Type 2I/R9 (LGMD2I/R9)
- AB-1003 is being studied in a P-I/II trial (LION-CS101) against PBO to evaluate safety in adults aged 18–65 years with LGMD2I/R9. Recruitment is underway following the dosing of the first patient
- AB-1003 uses an AAV vector to transfer a healthy FKRP gene, a common approach in gene therapy due to AAVs' safety, non-replicating nature, and ease of engineering.
MB-108

- The US FDA has granted ODD to MB-108 for the treatment of malignant glioma
- MB-108 is under investigation in a P-I trial, with patient enrollment ongoing at the University of Alabama at Birmingham
- MB-108 is a herpes simplex virus type 1 (HSV-1) oncolytic virus designed to target and treat malignant glioma.
IMSB301
- The US FDA has granted ODD and RPDD to IMSB301 for the treatment of cGAS-driven Type I interferonopathy, AGS
- IMSB301 is under evaluation in a P-I study among healthy subjects, with plans to advance into P-Ib/II trials targeting AGS, lupus, and other cGAS-driven conditions
- IMSB301 is an oral small molecule that inhibits the cGAS enzyme, blocking cGAMP production to prevent pathological inflammation.
BBM-D101
- The US FDA has granted ODD and RPDD to BBM-D101 for the treatment of DMD
- The therapy uses recombinant AAV technology to deliver an optimized gene expression cassette to muscle tissues for DMD treatment
- BBM-D101 is currently in an early P-I investigator-initiated study assessing its safety and efficacy in DMD patients.
RAG-21

- The US FDA has granted ODD to RAG-21 for the treatment of amyotrophic lateral sclerosis (ALS)
- RAG-21 uses RNA interference (RNAi) via the SCAD platform to target FUS mRNA, reducing toxic protein levels and addressing FUS-ALS pathology. Preclinical data suggest it prevents motor neuron degeneration and improves outcomes in aggressive ALS subtypes.
Deramiocel
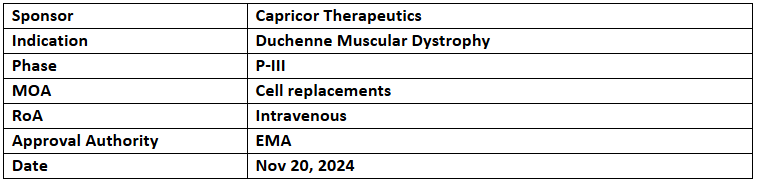
- The EMA granted ODD and ATMP designations to Deramiocel for the treatment of DMD
- Capricor has initiated a rolling BLA submission to the US FDA for full approval of Deramiocel in patients with DMD-cardiomyopathy, with complete submission planned by YE’24
- Deramiocel (CAP-1002) comprises allogeneic cardiosphere-derived cells (CDCs) that have shown immunomodulatory, antifibrotic, and regenerative effects in dystrophinopathy and heart failure.
CBL-514
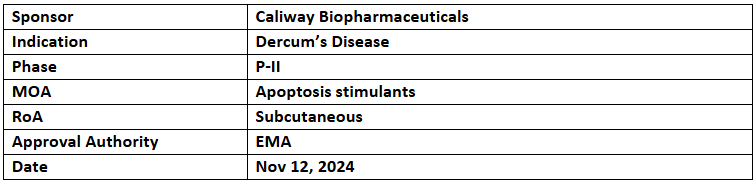
- The EMA has granted ODD to CBL-514 for the treatment of Dercum’s disease, offering 10 years of market exclusivity upon approval. The US FDA also granted FTD and ODD for the same indication
- Caliway has completed P-II (CBL-0201DD) trial showed a dimension reduction of >50% in 64.5% of painful lipomas, with 38.7% achieving complete clearance and a 4.7-point reduction in pain
- A P-IIb (CBL-0202DD) trial is underway to evaluate the safety and efficacy of CBL-514 vs. PBO in Dercum’s disease, with results anticipated by Q4’25. A global P-III trial for non-surgical fat reduction is planned for 2025
- CBL-514, a first-in-class small-molecule lipolysis injection, reduces subcutaneous fat by inducing adipocyte apoptosis and lipolysis, with no systemic side effects on the central nervous, cardiovascular, or respiratory systems.
OCU410ST
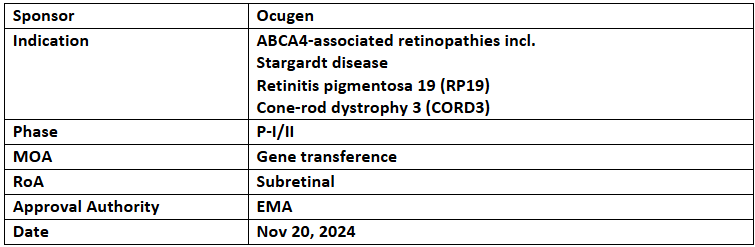
- The EMA has granted Orphan Medicinal Product Designation to OCU410ST for the treatment of ABCA4-related retinopathies such as Stargardt disease, retinitis pigmentosa 19 (RP19), and cone-rod dystrophy 3 (CORD3). The US FDA granted ODD for the same in Apr 2023
- Dosing in the P-I GARDian trial for Stargardt disease has concluded, demonstrating favorable safety. The DSMB has approved advancing to P-II, with preliminary data showing an 84% reduction in atrophic lesion growth at 6 months. Ocugen plans to pursue an accelerated MAA for OCU410ST
- OCU410ST employs an AAV platform to deliver the RORA gene, with Ocugen's modifier gene therapy approach. This strategy leverages Nuclear Hormone Receptors (NHRs) to regulate key physiological functions, including photoreceptor development, metabolism, phototransduction, inflammation, and cell survival.
ExoPTEN
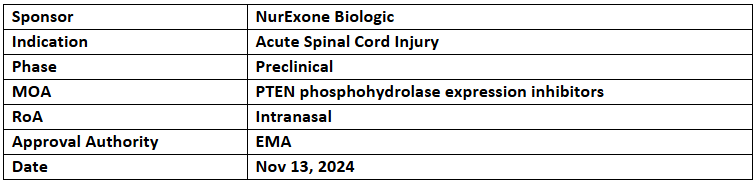
- The EMA has granted Orphan Medicinal Product Designation to ExoPTEN for the treatment of acute spinal cord injury.
- ExoPTEN comprises exosomes loaded with a specific siRNA sequence as the active pharmaceutical ingredient (API). Preclinical studies demonstrated nerve regeneration, regrowth, and functional recovery with intranasal administration. ExoPTEN is under development for clinical evaluation to address spinal cord injuries.

Gildeuretinol (ALK-001)
- The US FDA has granted RPDD and Fast Track Designation (FTD) to Gildeuretinol (ALK-001) for the treatment of Stargardt disease. The company plans to apply for a Priority Review Voucher alongside NDA submission
- In the TEASE-1 study, Gildeuretinol reduced the growth rate of atrophic retinal lesions by 21.6% at 24 months, with a 29.5% reduction observed. The growth rates were 0.18 mm/year with Gildeuretinol vs. 0.23 mm/year with PBO
- Safety data from TEASE-1 and TEASE-3 showed favorable results with no AEs linked to hyper or hypo-vitaminosis A, such as xerophthalmia, chromatopsia, delays in dark adaptation, or night blindness
- Gildeuretinol acetate is an oral therapy designed to reduce vitamin A dimerization without affecting the visual cycle. Preclinical studies demonstrated its ability to normalize vitamin A dimerization and prevent retinal degeneration and vision loss in Stargardt disease models.
LBS-007
- The US FDA has granted FTD to LBS-007 for the treatment of acute myeloid leukemia (AML).
- LBS-007 is being evaluated in a P-I/II dose-escalation and expansion trial to assess safety and tolerability in relapsed or resistant acute leukemias.
- LBS-007 a natural, non-ATP cell cycle inhibitor, targets CDC7 kinase activity, inhibiting tumor proliferation and inducing cancer cell death. Preclinical studies have shown potent activity against both leukemia and solid tumors.
ELA026
- The US FDA has granted FTD to ELA026 for the treatment of secondary hemophagocytic lymphohistiocytosis (sHLH).
- ELA026 is a first-in-class antibody therapy targeting signal regulatory protein alpha (SIRPα) to address secondary HLH. ELA026 offers a novel therapeutic approach to a life-threatening condition.
GNSC-001

- The US FDA has granted FTD to GNSC-001 for the treatment of osteoarthritis (OA) of the knee
- In a P-I study, GNSC-001 (intra-articular) demonstrated tolerability, increased IL-1Ra levels in synovial fluid for 12 months, trends toward improved pain and function scores, and limited disease progression
- GNSC-001 is an AAV-based gene therapy delivering optimized IL-1Ra to block IL-1 signaling, addressing OA-related inflammation, pain, and cartilage damage with long-term relief via a single joint injection.
VAD044
- The US FDA has granted FTD to VAD044 for the treatment of hereditary hemorrhagic telangiectasia (HHT)
- VAD044, an oral, once-daily allosteric AKT inhibitor, is being studied under a P-Ib trial for this indication. VAD044 represents a promising treatment approach for patients with HHT.
FLQ-101
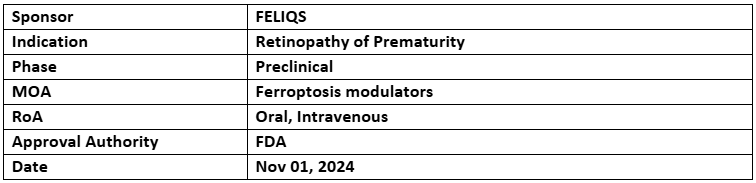
- The US FDA has granted FTD to FLQ-101 for preventing retinopathy of prematurity (ROP)
- The P-Ib/II (tROPhy-1) trial of FLQ-101 in the US and Japan is anticipated to begin in Q1 2025. FLQ-101 promotes retinal vascularization and mitigates inflammation and abnormal neovascularization
- FLQ-101 is an oral/IV solution that promotes retinal vascularization and protects against inflammation and abnormal neovascularization. FLQ-101 received ODD in 2024. Additionally, the company plans to submit an IND for FLQ-104 for intermediate dry-AMD in H2 2025.
ALE.P02
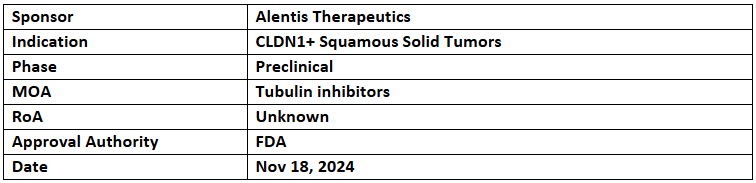
- The US FDA has granted FTD to ALE.P02 for the treatment of advanced or metastatic CLDN1+ squamous solid tumors
- The FDA has cleared the IND application for a P-I/II study in CLDN1+ squamous solid tumors, expected to begin in Q1 2025
- ALE.P02, a first-in-class ADC, combines a tubulin inhibitor with an antibody targeting the CLDN1 epitope on cancer cells, offering a novel approach to targeted therapy.

Libevitug (HH-003)
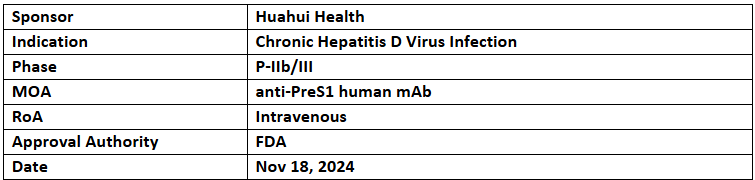
- The US FDA has granted Breakthrough Therapy Designation (BTD) to Libevitug (HH-003) for the treatment of chronic HDV infection
- The designation was based on results from two studies: HH003-201 (NCT05674448; presented at EASL 2023) and the ongoing HH003-204 (NCT05861674), with results pending publication
- Libevitug is a novel antibody targeting the pre-S1 domain of the HBV/HDV envelope protein. It prevents viral binding to the NTCP receptor, blocking hepatocyte entry and neutralizing HBV/HDV infection.
Nipocalimab

- The US FDA has granted BTD to Nipocalimab for the treatment of moderate-to-severe Sjögren’s disease (SjD)
- The designation was based on the P-II DAHLIAS study, which evaluated Nipocalimab (5 or 15 mg/kg, IV, Q2W) versus PBO in 163 adults with active primary SjD over 22 weeks alongside SoC. Data was presented at EULAR 2024
- Nipocalimab is an investigational mAb designed to block FcRn, reducing circulating immunoglobulin G (IgG) levels while preserving other immune functions. A P-III trial is ongoing, based on P-II results for Nipocalimab.
Ifebemtinib + Garsorasib
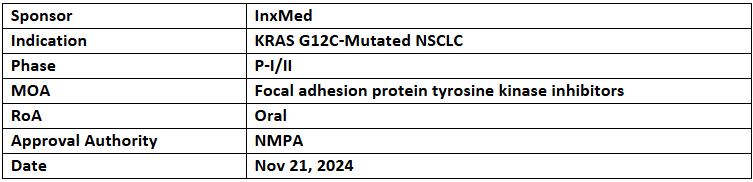
- The NMPA has granted BTD to Ifebemtinib (IN10018) plus Garsorasib as a 1L treatment for KRAS G12C-mutated NSCLC. InxMed is actively seeking global partnerships to advance the development of Ifebemtinib
- The designation was based on results from a P-Ib/II trial, demonstrating an ORR of 90.3%, DCR of 96.8%, and mPFS not reached. The study reported 28 confirmed PRs and 2 SDs among 31 efficacy-evaluable patients. Synergistic effects were observed with other cancer treatments, including anti-PD-(L)1 antibodies and RAS inhibitors
- InxMed is conducting a registrational trial for Pt-resistant ovarian cancer, with plans to submit an NDA to the NMPA in 2025. Additionally, PoC studies are ongoing in lung, colorectal, melanoma, and pancreatic cancers, with some progressing to pivotal trials
- Ifebemtinib is a selective, orally administered, small molecule inhibitor for focal adhesion kinase, which has demonstrated significant clinical synergies with targeted therapies, immunotherapies, and standard chemotherapies.

Lisaftoclax (APG-2575)
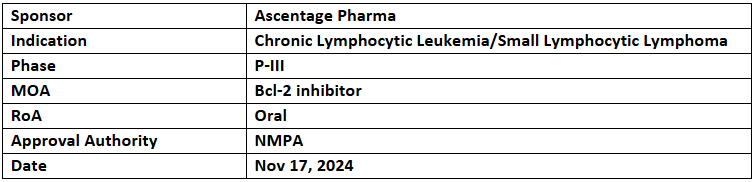
- The NMPA has accepted and granted Priority Review to the NDA for lisaftoclax (APG-2575) for relapsed/refractory (r/r) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL)
- The decision is based on a registrational P-II Chinese trial (APG2575CC201) with the 1EP of overall response rate (ORR)
- Lisaftoclax is a Bcl-2 selective inhibitor designed to block the antiapoptotic protein Bcl-2, thereby re-establishing normal apoptosis in cancer cells.

Ifetroban
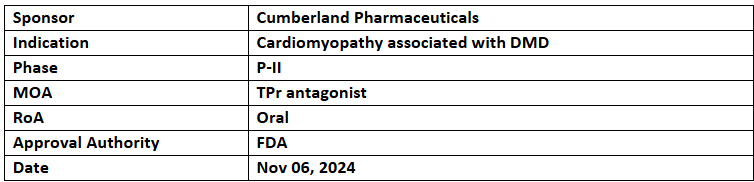
- The US FDA has granted ODD and RPDD to Ifetroban for the treatment of cardiomyopathy associated with Duchenne muscular dystrophy (DMD)
- The company is conducting an additional P-II study (FIGHTING FIBROSIS) to assess the safety and efficacy of Ifetroban compared to placebo (PBO) in patients with idiopathic pulmonary fibrosis (IPF) over a 52-week treatment period
- Ifetroban (QD, oral) is a selective thromboxane-prostanoid receptor (TPr) antagonist currently being evaluated in a P-II study (FIGHT DMD) for its safety, efficacy, and pharmacokinetics (PK) in patients with DMD, with results expected in Q4 2024.
Gildeuretinol (ALK-001)
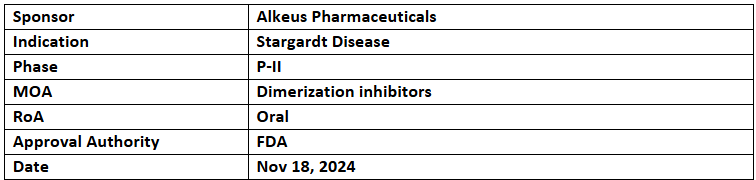
- The US FDA has granted RPDD and FTD to Gildeuretinol (ALK-001) for the treatment of Stargardt disease. The company plans to apply for a Priority Review Voucher alongside NDA submission
- In the TEASE-1 study, Gildeuretinol reduced the growth rate of atrophic retinal lesions by 21.6% at 24 months, with a 29.5% reduction observed. The growth rates were 0.18 mm/year with Gildeuretinol vs. 0.23 mm/year with PBO
- Safety data from TEASE-1 and TEASE-3 showed favorable results with no AEs linked to hyper or hypo-vitaminosis A, such as xerophthalmia, chromatopsia, delays in dark adaptation, or night blindness
- Gildeuretinol acetate is an oral therapy designed to reduce vitamin A dimerization without affecting the visual cycle. Preclinical studies demonstrated its ability to normalize vitamin A dimerization and prevent retinal degeneration and vision loss in Stargardt disease models.
AB-1003
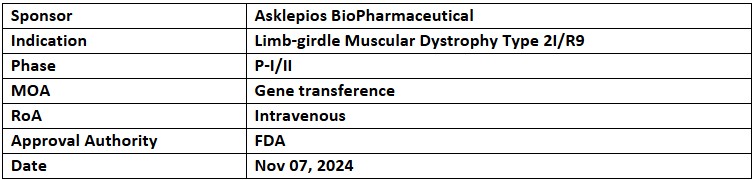
- The US FDA has granted ODD and RPDD to AB-1003 (LION-101) for the treatment of Limb-Girdle Muscular Dystrophy Type 2I/R9 (LGMD2I/R9)
- AB-1003 is being studied in a P-I/II trial (LION-CS101) against PBO to evaluate safety in adults aged 18–65 years with LGMD2I/R9. Recruitment is underway following the dosing of the first patient
- AB-1003 uses an AAV vector to transfer a healthy FKRP gene, a common approach in gene therapy due to AAVs' safety, non-replicating nature, and ease of engineering.
Elraglusib
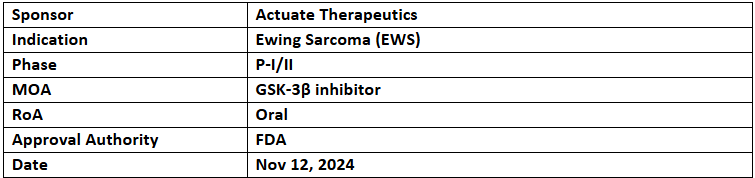
- The US FDA has granted RPDD to Elraglusib for the treatment of Ewing Sarcoma (EWS), making the company eligible for a Priority Review Voucher upon approval
- The P-I/II (Actuate-1902) study evaluates Elraglusib ± irinotecan ± temozolomide/cyclophosphamide + topotecan in pediatric patients with r/r EWS and related sarcomas. Topline data are expected in H2’25
- Elraglusib (9-ING-41) inhibits GSK-3β, a key target in cancer growth and resistance, with potential applications in the treatment of various cancers by modulating tumor signaling and immune response.
IMSB301
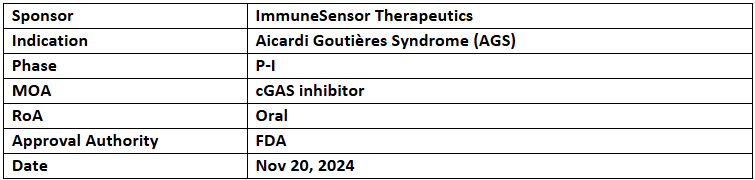
- The US FDA has granted ODD and RPDD to IMSB301 for the treatment of cGAS-driven Type I interferonopathy, AGS
- IMSB301 is under evaluation in a P-I study among healthy subjects, with plans to advance into P-Ib/II trials targeting AGS, lupus, and other cGAS-driven conditions
- IMSB301 is an oral small molecule that inhibits the cGAS enzyme, blocking cGAMP production to prevent pathological inflammation.
BBM-D101
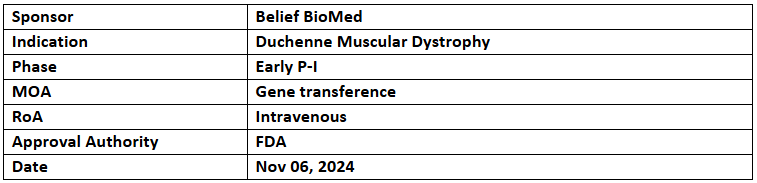
- The US FDA has granted ODD and RPDD to BBM-D101 for the treatment of DMD
- The therapy uses recombinant AAV technology to deliver an optimized gene expression cassette to muscle tissues for DMD treatment
- BBM-D101 is currently in an early P-I investigator-initiated study assessing its safety and efficacy in DMD patients.
AVG-002
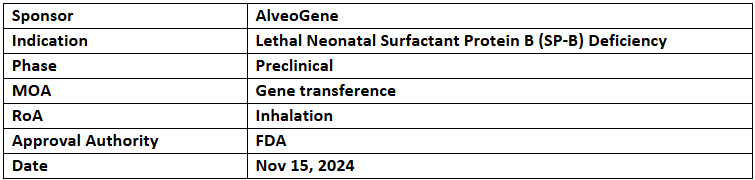
- The US FDA has granted RPDD to AVG-002 for the treatment of lethal neonatal SP-B deficiency
- Preclinical studies demonstrated prolonged survival in SP-B knockout models with a single dose of AVG-002, restoring normal lung function. AlveoGene aims for marketing authorization by 2028
- AVG-002 employs the InGenuiTy platform to deliver a functional SP-B gene to alveolar regions via a pseudotyped lentiviral vector for efficient respiratory instillation.

Selective Cytopheretic Device
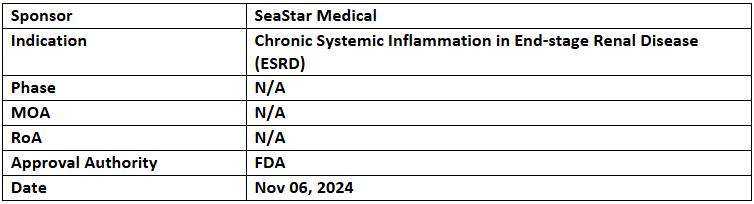
- The US FDA has granted Breakthrough Device Designation (BDD) to the Selective Cytopheretic Device (SCD) for the treatment of chronic systemic inflammation in ESRD patients undergoing chronic hemodialysis
- The SCD is a patented extracorporeal device employing immunomodulating technology to target proinflammatory cells, reducing hyperinflammation during continuous renal replacement therapy (CRRT).
STARgraft
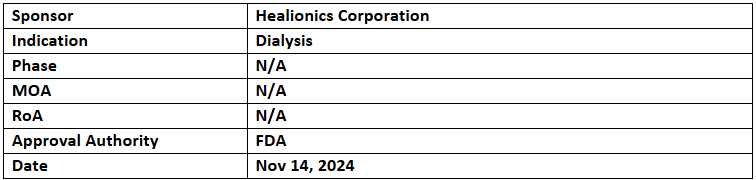
- The US FDA has granted BDD to STARgraft, an arteriovenous graft developed to ensure reliable bloodstream access for patients with kidney failure
- STARgraft, based on proprietary synthetic biomaterial technology, is designed to maintain sufficient flow rates for dialysis and reduce the risk of occlusion and infection.
EEG Implant System
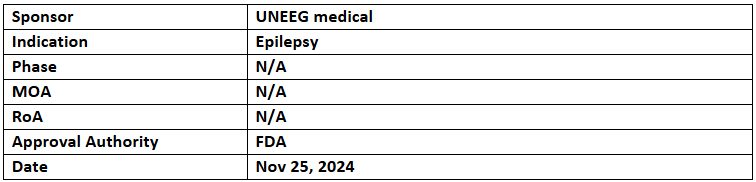
- The US FDA has granted BDD to the EEG Implant System for monitoring and diagnosing epilepsy
- In addition to FDA BDD, UNEEG's implantable device and its 2nd-gen Bluetooth-assisted system have secured CE-mark approval from the EC and are ready for launch
- The subcutaneous EEG Implant System offers 24/7 brainwave recording through a compact implant, transmitting data to hospitals where AI software detects seizures, providing long-term insights for epilepsy management.

Nexiguran Ziclumeran (nex-z)
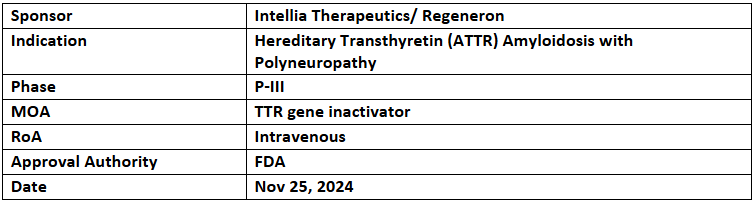
- The US FDA granted RMAT designation to nex-z for the treatment of hereditary transthyretin (ATTR) amyloidosis with polyneuropathy (ATTRv-PN)
- Nex-z (nexiguran ziclumeran or NTLA-2001) is an in-vivo CRISPR-based investigational therapy which inactivates the TTR gene, which codes for transthyretin protein, resulting in consistent, deep, and long-lasting TTR reduction, as demonstrated in interim P-I clinical data
- Development and commercialization of nex-z is led by Intellia as part of a multi-target collaboration with Regeneron.
Deramiocel (CAP-1002)
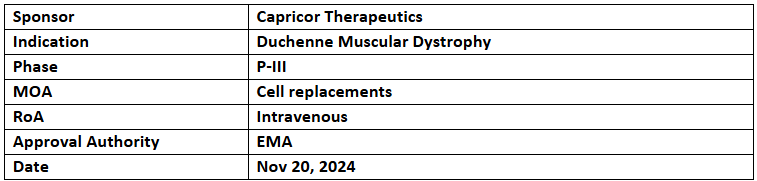
- The EMA granted ODD and ATMP designations to Deramiocel for the treatment of DMD
- Capricor has initiated a rolling BLA submission to the US FDA for full approval of Deramiocel in patients with DMD-cardiomyopathy, with complete submission planned by YE’24
- Deramiocel (CAP-1002) comprises allogeneic cardiosphere-derived cells (CDCs) that have shown immunomodulatory, antifibrotic, and regenerative effects in dystrophinopathy and heart failure.
References
- Leads Biolabs
- Cumberland Pharmaceuticals
- Leads Biolabs
- Asklepios BioPharmaceutical
- Mustang Bio
- ImmuneSensor Therapeutics
- Belief BioMed
- Ractigen Therapeutics
- Capricor Therapeutics
- Caliway Biopharmaceuticals
- Ocugen
- Alkeus Pharmaceuticals
- Lin BioScience
- Electra Therapeutics
- Vaderis Therapeutics
- Alentis Therapeutics
- Huahui Health
- Johnson & Johnson
- InxMed
- Ascentage Pharma
- Asklepios BioPharmaceutical
- Actuate Therapeutics
- ImmuneSensor Therapeutics
- AlveoGene
- SeaStar Medical
- Intellia Therapeutics
- Globenewswire
- PR Newswire
- Businesswire
- EINPresswire
- Accesswire
Related Post: New Drug Designations - October 2024

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














